Diversion and Dispersion
The FedInvent Portfolio | Taxpayer Funded Patent Applications for March 3, 2022
Hello from FedInvent,
On Thursday, March 3, 2022, the U.S. Patent Office published 9,874 pre-grant patent applications. One hundred ninety-nine (199) benefitted from taxpayer funding.
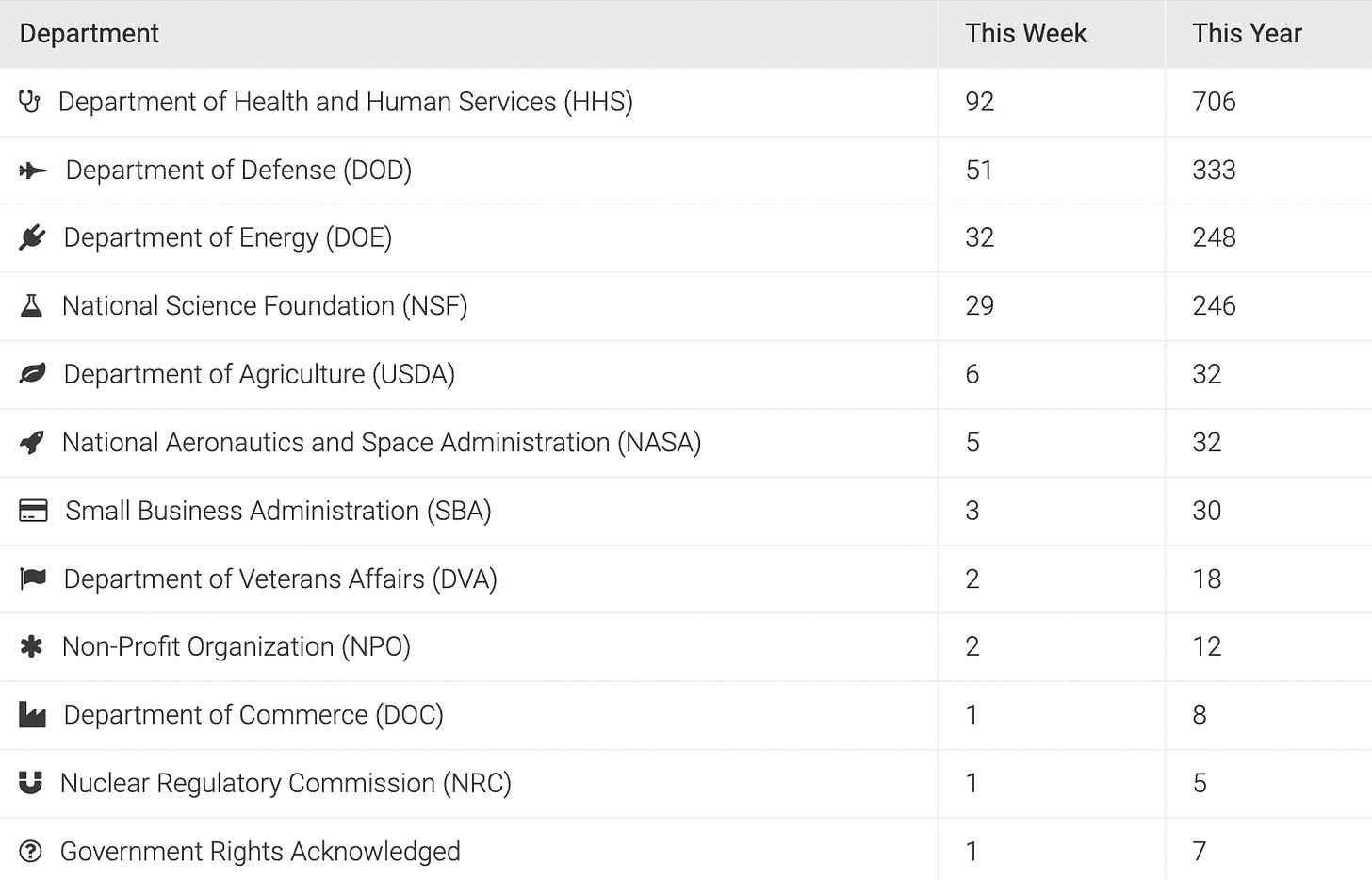
For the first nine weeks of this year, there are 1524 patent applications that had 1714 federal funding citations. The big four — HHS, DOD, NSF, and DOE — account for 1528 or 89% of the grants, contracts, agreements, or government assignees indicating taxpayer funding on these applications. That’s a lot of taxpayer funding.
The FedInvent Report for this week's application is available here. To browse by department, start here. Big button below.
We took a look at two of this week’s patent applications that illustrate the outcomes of policy-driven research and development. The first deals with the challenges the pharmaceutical industry has with drug diversion, a fancy name for healthcare workers stealing drugs, and tracking controlled substances and sensitive medicines inside complex medical facilities. The second application funded by Power America illustrates the challenge of analyzing taxpayer-funded R&D cash dispersion. Federal R&D cash dispersion is the art, science, and mechanics of getting federal money into the hands of researchers and developers who can create new innovations.
This week we came up short on interesting drawings. The drug diversion-related patent application was basically a still-life with flow chart invention. The Power America patent application’s drawings are full of electronic squiggly lines and phase angle charts. So we decided to highlight one of the beautiful drawings made without computer-aided design tools.
The first application we’ve included in the newsletter is about saving money (and lives). The second is about giving money away, Hence the vintage cash register image.
Stopping Drug Diversion
Drug diversion is a serious issue. Anyone who had been watching House reruns while waiting out the COVID-19 lockdowns knows how it works. Healthcare professionals redirect sensitive drugs for personal use or sale in the illegal drug market. Order twelve pills from the hospital pharmacy, give the patient ten. Take two. Bill the insurance company for all twelve.
Drug diversion and control of sensitive drugs is an issue for the Federal Drug Administration and the Federal Trade Commission and the insurance industry that pays for these controlled substances.
On November 27, 2013, the Drug Supply Chain Security Act (DSCSA) (Title II of Pub. L. 113–54) was signed into law. The DSCSA outlines critical steps to build an electronic, interoperable system by November 27, 2023, that will identify and trace certain prescription drugs as they are distributed within the United States. Another complex supply chain issue for the pharmaceutical industry.
We learned a lot about the medical supply chain back in December of 2020 as we watched the first shipments of Pfizer's COVID-19 vaccines from Pfizer's manufacturing facilities into the hands of UPS and FedEx. There were issues with controlling the drugs to prevent counterfeits from entering the drug supply chain. There were also lots of interesting logistics for the cold chain super cold refrigeration required to store the drugs until the doses were ready to go into waiting arms of Americans who wanted to get out of the house. We also learned that dry ice manufacturers in the U.S. made a killing.
According to the U.S. Department of Justice National Drug Intelligence Center (NDIC), the estimated cost of diversion to public and private medical insurers is more than $72 billion per year. Clinical drug diversion results in over $450M annually losses to healthcare systems. Clinical drug diversion is done by doctors, technicians, nurses, pharmacists, and others in the inside-the-healthcare-system supply chain.
This week Autonomous Healthcare's pre-grant patent application 20220068501, "System and Method for Tracking Sensitive Medications," was published on Thursday. Their invention provides a "last mile" solution for drug supply chain security. Inventors from Autonomous Healthcare present a way to use blockchain and biometrics to implement asset tracking for sensitive drugs. Sensitive medication packs are tracked along the healthcare facility supply chain, starting from where the sensitive medication is stored to the point of administration to the patient or in situations where the medication isn't given to a patient to its waste and deliberate destruction endpoint.
The National Institute on Drug Abuse (NIDA) at NIH funded this research.
Autonomous Healthcare's use of blockchain as the mechanism for tracking these substances provides an immutable and secure approach. Their invention provides a "last mile" solution for drug supply chain security. Their challenge will be to integrate their solution into the electronic health record systems and medication management platforms already in use within hospitals and healthcare facilities.
A Power America Patent Application
Sometimes we come across patent applications that contain the Bayh-Dole required regulatory data -- the federal agency and the contract number -- but the data isn't useable. One of those patent applications appeared this week. The government interest statement said that the inventors received funding from Power America. So the research began.
On January 15, 2014, the Obama Administration announced the creation of the Next Generation Power Electronics Institute, part of the administration's advanced manufacturing initiative. PowerAmerica is a private-public partnership between the U.S. Department of Energy, industry, and academia to advance wide bandgap (WBG) semiconductor manufacturing and accelerate the adoption of WBG semiconductor power electronics applications. The press release announced that the partner that would receive the Department of Energy government funding was North Carolina State University.
The Power America | Next Generation Power Electronics Institute provides the innovation infrastructure to support new product and process technologies, education, and training to become a global center of excellence for developing wide bandgap semiconductor devices and industry-relevant processes.
Wide-bandgap semiconductors permit devices to operate at much higher voltages, frequencies, and temperatures than conventional semiconductor materials like silicon and gallium arsenide. They are the key component used to make green and blue LEDs and lasers. The wider bandgap is particularly important for allowing devices that use them to operate at much higher temperatures, on the order of 300 °C. The ability to operate at high temperatures makes the WBG semiconductors highly attractive for military applications. WBG technology is one of the leading contenders for next-generation devices for general semiconductor use.
Like most policy-driven research and development, there was a competition for creating the institute. A team led by North Carolina State University won.
North Carolina State University brought together a consortium of leading companies that included some of the world's leading wide bandgap semiconductor manufacturers, leading materials providers, and critical end-users like John Deere and Delphi. The NC State team included universities on the cutting edge of technology development and research, all in a vibrant and entrepreneurial region that serves as the foundation for ongoing U.S. leadership in this important technology. The Department of Energy is awarding $70 million over five years, matched by at least $70 million in non-federal commitments by the winning team of businesses and universities, along with the state of North Carolina.
The Power America | Next Generation Power Electronics Institute cooperative research agreement is part of the Department of Energy Office of Energy Efficiency and Renewable Energy (EERE) portfolio.
Power America uses Member Initiated Projects (MIP) to establish a process for supporting technology development that is focused on pre-competitive yet critical needs of the broad wide bandgap community. Entities can compete for funding for projects defined by Power America members. The request for proposal documents read like an SBIR grants.gov funding opportunity with a little bit of Broad Agency Announcement area of interest language thrown in. Proposals are accepted from members and non-members. Non-members who submit proposals must join PowerAmerica if their proposal is selected for funding.
In 2014 the partnership's members included seven universities and labs and 18 companies. Today Power America counts 18 universities, five national labs, and 45 companies as members. Power America is focused on advances in the Silicon Carbide and Gallium Nitride power electronics ecosystem. The organization claims to have filed 33 invention disclosures to date.
This week, Virginia Tech's Power America funded patent application 20220065950, "Core Loss Characterization and Measurement," was published. This patent application is the latest contribution to the 11 published patent applications and eight patents funded under the DOE-NC State Power America cooperative research agreement. Some inventors and Power America members report their taxpayer funding from the Energy Efficiency and Renewable Energy (EERE) office at the U.S. Department of Energy. These applications are easier to track. Other inventors report their taxpayer-funding in their patent application's government interest statement using N.C. State generated sub-award numbers. Not so easy to find and attribute to the correct federal agency.
So the patent and published application count for Power America-funded research is fluid.
Patent Applications By the Numbers
On Thursday, March 3, 2022, the U.S. Patent Office published 9,874 pre-grant patent applications. A big week. One hundred ninety-nine (199) benefitted from taxpayer funding. Here is how March 3rd's patent applications break down.
One hundred ninety-two (192) patent applications have Government Interest Statements.
Thirty-one (31) applications have an applicant or an assignee that is a government agency.
A federal department is the only assignee on nine (9) patent applications.
The 199 new patent applications have 225 department-level funding citations.
These applications are the work of 692 inventors.
The 662 American inventors come from 38 states and the District of Columbia.
The thirty (30) foreign inventors come from 14 countries.
There are 137 patent applications (69%) where at least one assignee is a college or university, the HERD.
Sixteen patent applications (16) resulted from the collaboration between two or more universities.
Federally Funded Research and Development Centers (FFRDCs) received 17 patent applications. This week included
Four (4) patent application is assigned a Y CPC symbol indicating that the invention may be useful in mitigating the impact of climate change.
Top Three States This Week
California has 31 first-named inventors and 114 total inventors.
Massachusetts has 24 first-named inventors and 104 total investors.
Illinois has 16 first-named inventors and 62 total inventors.
Count By Department
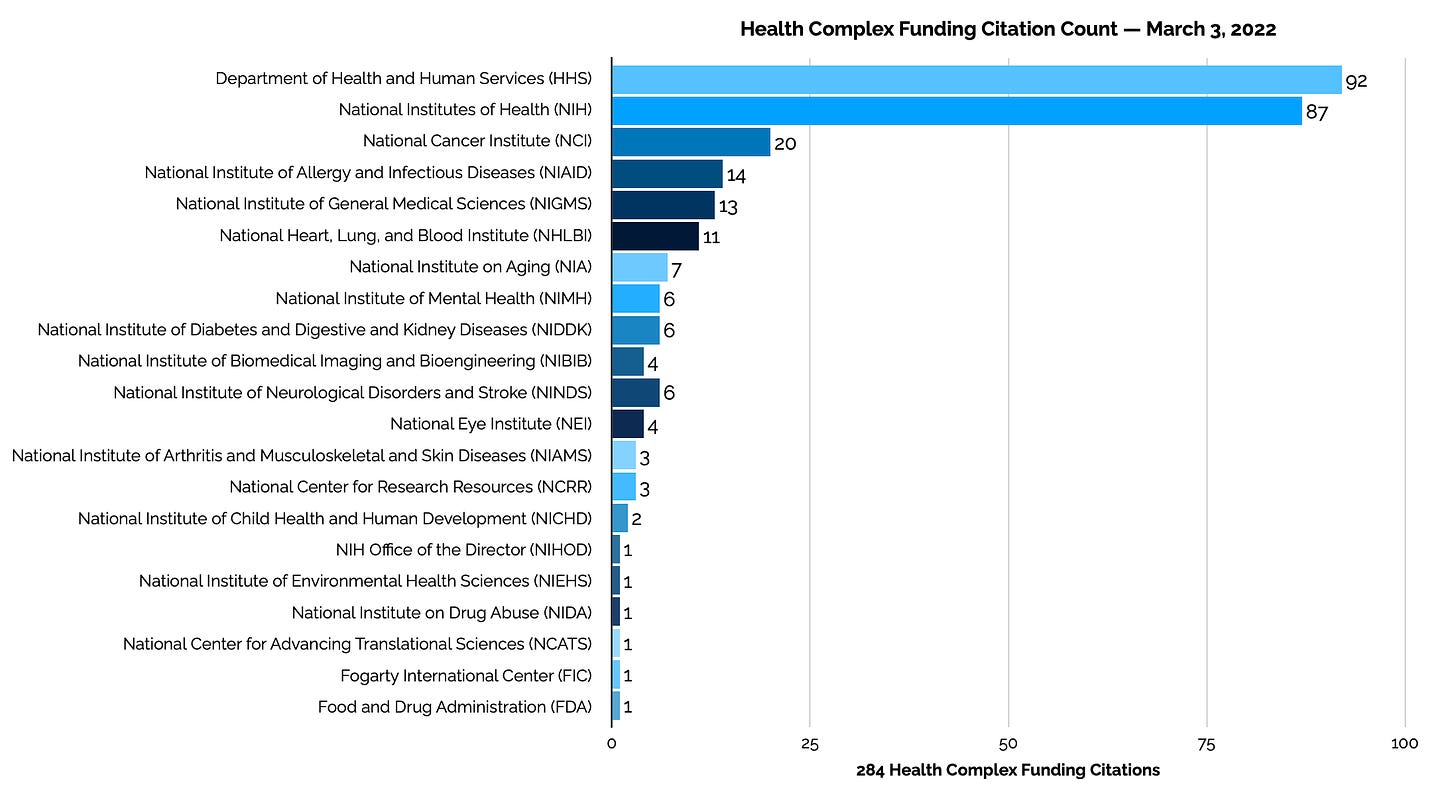
Health Complex This Week
The Health Complex section of the FedInvent Report presents new patent applications from the Department of Health and Human Services and its parts. Each week the Health Complex has the highest number of published pre-grant taxpayer-funded patent applications.
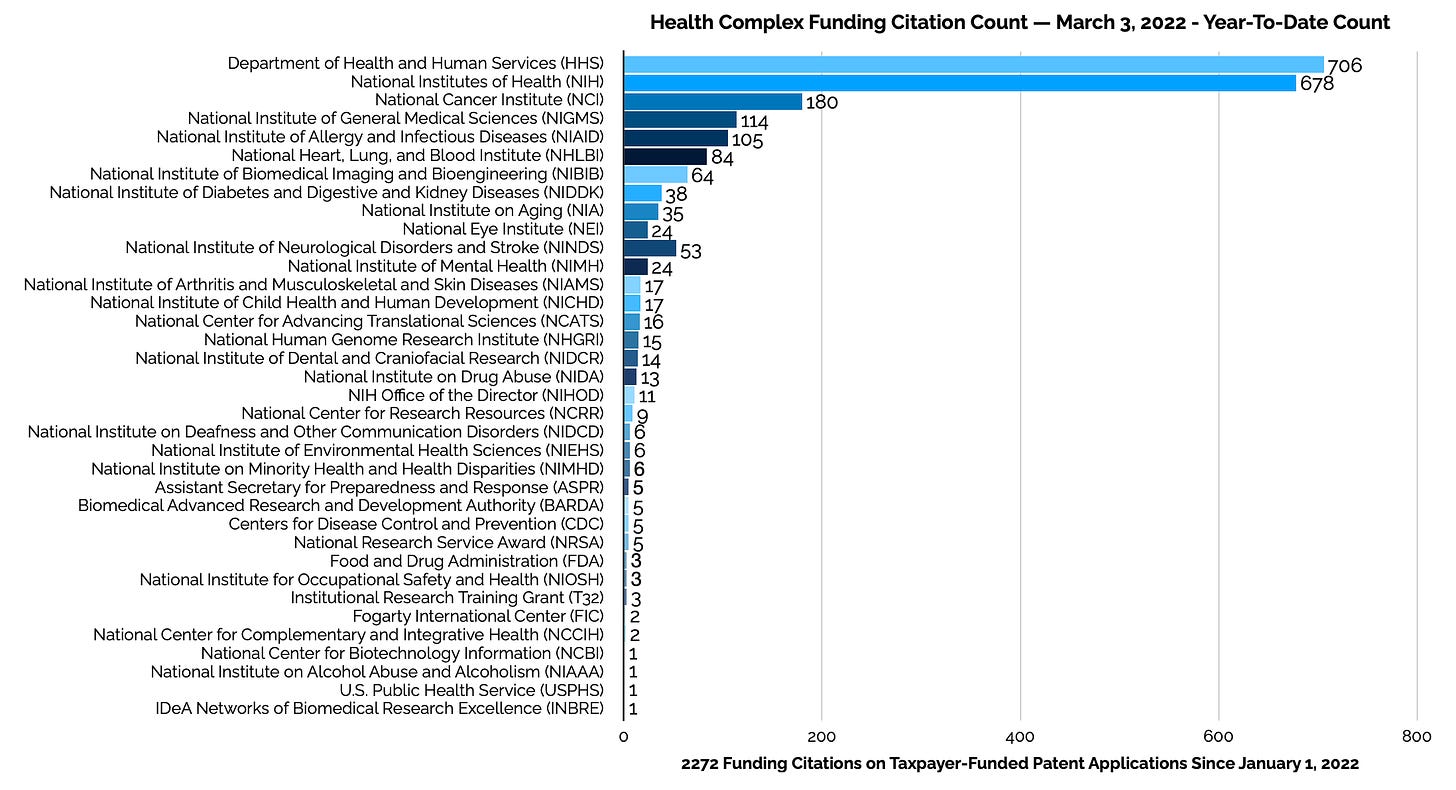
Health Complex Year To Date
The Health Complex's inventors have included 2,272 individual funding citations on the 706 pre-grant patent applications published since the beginning of 2022.
Before We Go
Like most people, we've been watching the war in Ukraine unfold. We've been receiving on the ground reporting from people on the ground. We are getting dispatches from Gesher Galicia, a non-profit organization focused on Jewish genealogical and historical research on Galicia, formerly a province of Austria-Hungary and today an area divided between southeastern Poland and western Ukraine.
We are publishing the messages on a page called Messages from Ukraine. The goal is to get real reports of what is happening in Ukraine to as many people as possible. We'll update the page as the messages come in. So please check back for updates and share the page with as many people as possible. Thanks in advance for your help.
We're still working on our reporting on Federally Funded Research and Development Centers and DOD's University Affiliated Research Centers. Like all things with patents and patent applications, things are taking time to get it right. Please stay tuned.
Thank you for reading FedInvent. Please share the newsletter and the report with people interested in policy-driven science and the inventions and innovations it creates. Please spread the word.
We'll be back with the latest reporting on taxpayer-funded patents later this week.
The FedInvent Team
FedInvent tells the stories of inventors, investigators, and innovators. Wayfinder Digital's FedInvent Project follows the federal innovation ecosphere, taxpayer money, and the inventions it pays for. FedInvent is a work in progress. Please reach out if you have questions or suggestions. You can reach us at info@wayfinder.digital.