Battling COVID the Spike Protein
Taxpayer Funded Patent Applications Published November 18, 2021
Good Afternoon from FedInvent,
On Thursday, November 18, 2021, the Patent Office published 8,006 pre-grant patent applications. One hundred seventy-three (173) benefitted from taxpayer funding.
The FedInvent Patent Application Report is here. You can browse the patent application details by federal department here.
This week's highlights include:
New COVID-19-Related Inventions
PPE for Diagnostic Imaging Healthcare Workers
An Antibody for a Future Epidemic
An International Collaboration To Beat MERS
Antibodies for Hemophilia
HIV-En Discoveries
The AstraZeneca SARS-CoV-2 Antibody Application
This week's patent application includes an antibody application from AstraZeneca (20210355196). A team of scientists led by Mark T. Esser, Vice President and Head of Microbial Sciences, BioPharmaceuticals R&D invented the antibody. The monoclonal antibody compound for treating the COVID-19 virus was discovered at Vanderbilt University Medical Center (VUMC) and developed by AstraZeneca. The original antibodies that were the basis for the engineered long-acting antibodies that make up the AZD7442 two-antibody cocktail were isolated last year at VUMC.
On November 18, 2021, AstraZeneca announced that new clinical trial results showed its antibody treatment is highly effective at preventing Covid-19 in people who may not respond well to vaccines. The data showed that patients given a single injection of the antibody treatment, known as AZD7442, were 83% less likely to develop symptomatic cases of the coronavirus than participants who were given a placebo.
The VUMC research was supported by the National Institute of Allergy and Infectious Diseases, part of the National Institutes of Health, the Defense Advanced Research Projects Agency of the U.S. Department of Defense.
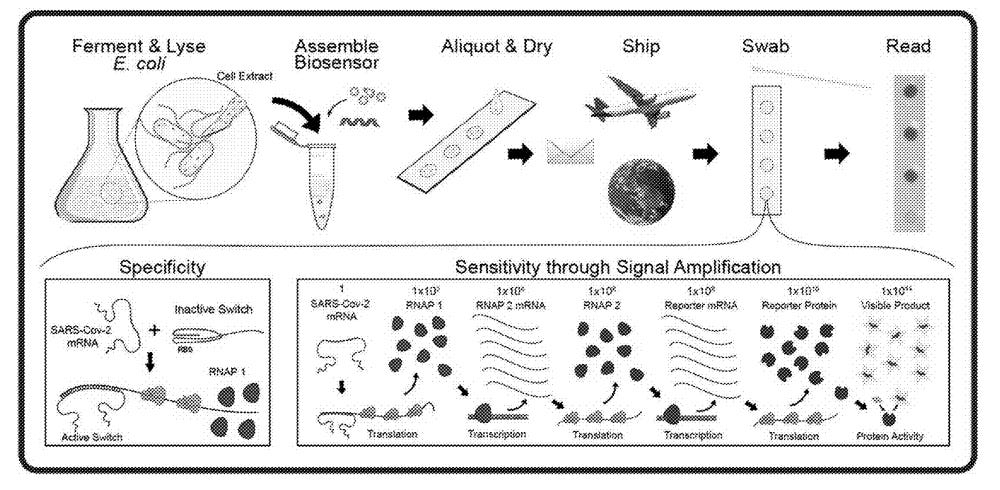
A Cost-Effective Test Kit
One of the biggest challenges to stopping the spread of the virus is "silent transmission" through infected individuals who have yet to show symptoms during the up-to-14-day incubation period or through asymptomatic carriers, as studies estimate that up to 88% of infected people do not exhibit symptoms. The lack of a simple inexpensive diagnostic test for the virus impedes measurement of the masses, which measurement will allow health planners to properly coordinate public health response to predicted and current rates of infection.
The patent application of inventors at Brigham Young University for "a simple paper-based test (similar to a pregnancy test), that can be completed at home, rapidly be scaled to 300+ million tests to test everyone in the country. The test enables the identification and quarantining of asymptomatic spreaders to more efficiently monitor and eventually eliminate the spread of the disease published on Thursday (20210355552). These tests could also facilitate daily testing by those who interact with high-risk individuals to prevent transmission and daily testing by high-risk individuals to catch the infection early and treat it early. In addition, testing will help prevent overwhelming our health care system and the limited number of ventilators available. Testing would be invaluable in airports to screen travelers quickly. It will also enable regular testing of patients and caregivers in care facilities or for children in schools. The key is a very inexpensive test that is simple, accurate, and easily scaled up for distribution.
National Institutes of Health, National Heart, Lung, and Blood Institute (NHLBI) funded this work.
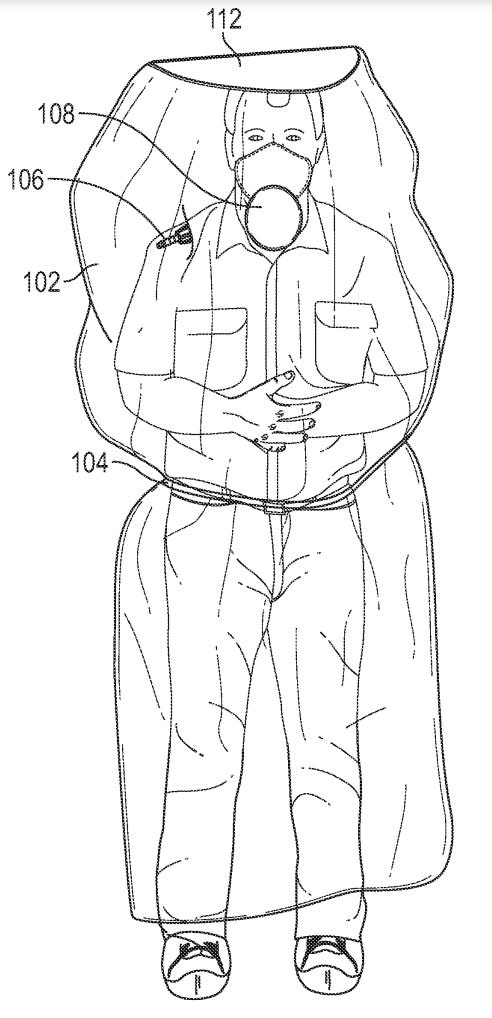
Personal Protective Equipment for Diagnostic Imaging
An interdisciplinary team of imaging experts working through NIH tackled another aspect of treating patients and protecting healthcare workers with COVID-19 and other infectious diseases. Application 20210353150, "Isolation Devices to Reduce Contamination During Imaging of Patients," presents disposable personal protective equipment (PPE). This equipment may be used to isolate patients and prevent cross-contamination of infectious diseases during medical imaging.
Another Approach to a COVID Vaccine
Northeastern University's application, 20210353743, "Biomaterial-Based COVID-19 Vaccine," for an oxygen-generating cryogel vaccine consisting of an antigen, a chemoattractant/stimulating factor for immune cells, an adjuvant, and an oxygen-producing compound was published on Thursday as well. The National Science Foundation funded this research.
More COVID-10 Spike Protein Inventions
University of Colorado's spike protein invention is 20210355170, "Stabilizing Mutants of Prefusion SARS-COV-2 (COVID-19) Spike Protein and Improved Yeast Surface Display Engineering Platform for the Same." The application is focused on modified viral fusion proteins having enhanced stability characteristics that may be useful for vaccine formulation, serological diagnostic testing, and enhancement of expression titers.
The University of Vermont and State Agricultural College's 20210353620, "Methods and Uses of Protein Disulfide Isomerase Inhibitory Compounds," presents a method of treating a severe acute respiratory syndrome (SARS) virus infection using a protein disulfide isomerase (PDI) inhibitor compound.
In Other COVID News
On November 18, 2021, Pfizer Inc., New York, New York, was awarded a $5,295,000,000 firm-fixed-price contract for 10 million doses of Pfizer's oral protease inhibitor drug PF-07321332. PF-07321332 (PAXLOVID) is an antiviral drug developed by Pfizer which acts as an orally active 3CL protease inhibitor. Fiscal 2021 CARES Act and Paycheck Protection Program and Health Care Enhancement Act obligated $5,295,000,000 at the time of the award. U.S. Army Contracting Command, Aberdeen Proving Ground, Maryland, is the contracting activity.
Pfizer will allow generic manufacturers to supply its promising Paxlovid pill to 95 low-and middle-income nations covering about 53% of the world's population.
On November 16, 2021. Glaxo Smith Kline LLC, Durham, North Carolina, was awarded a $651,094,500 firm-fixed-price contract for Sotrovimab Therapeutic to treat COVID-19. Sotrovimab, sold under the brand name Xevudy, is an investigational human neutralizing dual-action monoclonal antibody with activity against severe acute respiratory syndrome coronavirus 2, known as SARS-CoV-2. It is under development by GlaxoSmithKline and Vir Biotechnology, Inc.
Fiscal 2010 Defense Production Act funds in the amount of $651,094,500 were obligated at the time of the award. U.S. Army Contracting Command, Aberdeen Proving Ground, Maryland, is the contracting activity.
Monoclonal Antibodies for Past and Future Epidemics
The Future Epidemic
A collaboration of inventors from the Albert Einstein College of Medicine, the University of Texas at Austin, Adimab, Llc, and Mapp Biopharmaceutical resulted in 20210355195, "Anti-Crimean-Congo Hemorrhagic Fever Virus Antibodies, and Methods of Their Generation and Use," The inventors discovered, isolated, and characterized an extensive panel of CCHFV-specific monoclonal antibodies from the memory B cells of four CCHFV-convalescent donors.
Crimean-Congo Hemorrhagic Fever (CCHF) virus is a potentially fatal tick-borne viral disease; CCHF is on the WHO list for emerging infections likely to cause major epidemics in the near future. Early diagnosis of CCHF is very important for both patient treatment and infection control.
Transmission to humans occurs through contact with infected ticks or animal tissue, or blood. CCHF can also be transmitted from one infected human to another by contact with infectious bodily fluids. Documented spread of CCHFV has occurred in hospitals due to improper sterilization of medical equipment, reuse of injection needles, and contamination of medical supplies. According to the Centers for Disease Control and Prevention, CCHFV has a fatality rate of up to 50%.
MERS and Another Coronavirus
Middle Eastern Respiratory Syndrome (MERS) has been around since 2012. Middle East respiratory syndrome coronavirus (MERS-CoV) is a virus transferred to humans via infected dromedary camels. It is a zoonotic virus, meaning it is transmitted between animals and people, and it is contractable through direct or indirect contact with infected animals. MERS-CoV has been identified in dromedaries in several countries in the Middle East, Africa, and South Asia. Korea had its last case in 2018 when a business traveler returned home from a trip to Kuwait.
Inventors from the Korea Disease Control and Prevention Agency, formerly Korea Centers for Disease Control and Prevention, and the NIH collaborated to develop a monoclonal antibody binding specifically to the spike protein of Middle East respiratory syndrome coronavirus. Their invention is described in publication 20210355193, "Monoclonal Antibody for Spike Protein for Middle East Respiratory Syndrome Coronavirus and Use Thereof."
To Treat and Prevent HIV
The International AIDS Vaccine Initiative's application, 20210355197, "Anti-HIV Antibodies," is for monoclonal antibodies that bind to HIV Env. The Env protein is an entry machine built to bind to CD4, undergo a series of conformational changes, fuse the cell and viral membranes, and deliver the viral core to the cell's cytoplasm. That's medical speak for this is how HIV gets into cells. This invention may provide another weapon in the fight against HIV/AIDS.
The National Institutes of Health's application, "HIV-1 ENV Fusion Peptide Nanoparticle Carrier Conjugates and Their Use" (20210353740), was published this Thursday. This application is the result of intramural work by scientists at the Vaccine Research Center of the National Institute of Allergy and Infectious Diseases.
A Hemophilia Antibody
This Thursday, the University of Texas Health Science Center at Tyler contributed publication 20210355231, "Treatment and Prevention of Hemophilic Arthropathy With an Antibody Against Endothelial Cell Protein C Receptor (EPCR)," to the federal innovation ecosphere. This monoclonal antibody is for the treatment of hemophilic arthropathy. Hemophilic arthropathy is a debilitating disease caused by hemophilia. Repetitive hemarthroses and progressive joint damage characterize it. Hemophilic arthropathy has a significant negative impact on mobility and quality of life of people with Hemophilia.
In researching this patent application discovered the Blood Podcast published by the American Society of Hematology. (Everyone has a podcast.) You can listen to it here.
Patent Applications By The Numbers
On Thursday, November 18, 2021, the Patent Office published 8,006 pre-grant patent applications. One hundred seventy-three (173) benefitted from taxpayer funding.
Here is how the numbers came out this week:
One hundred sixty-six (166) patent applications have Government Interest Statements.
Twenty-five (25) have an applicant or an assignee that is a government agency.
The 173 applications have 203 department-level funding citations.
These applications are the work of 627 inventors.
The 595 American inventors come from 40 states, Puerto Rico, and the District of Columbia.
Thirty-two (32) foreign inventors come from 12 countries
There are 113 applications (65%) where at least one assignee is a college or university, the HERD.
A Federally Funded Research and Development Center (FFRDCs) is the assignee or applicant on nine (9) applications.
A federal department is the only of the assignees on 15 patents.
Patent Application Count By Department
The table below shows the number of patent applications citing funding by one or more Department Level entities.
The Health Complex
The following table shows the number of funding references citing the Department of Health and Human Services, the National Institutes of Health, and the individual Institutes.
That's this week's FedInvent patent applications update. The FedInvent Patent Applications Report has the data and the details on many more taxpayer-funded inventions.
The Thursday, November 25, 2021, FedInvent Patent Application Report links will be included in the Thursday, December 2, 2021, newsletter. We're sure you won't be reading about patents over the holiday weekend. We want to avoid the FedInvent family from giving us the evil eye because we are fooling around with patents while the turkey is in the oven.
As usual, there are many more taxpayer-funded patents than we can cover here. Please explore the FedInvent Patent Report. It's an important addition to your newsletter subscription.
If you'd like to catch up on earlier FedInvent Reports, you can access the newsletters here on Substack. The reports are available on the FedInvent Links page.
If you'd like to catch up on earlier FedInvent Reports, you can access the newsletters here on Substack. In addition, the reports are available on the FedInvent Links page.
If you aren't a paid subscriber yet, please consider subscribing. It will help us keep the lights on. You'll get your money's worth from the subscription.
We'll see you later this week with the FedInvent report on Thursday's published pre-grant patent applications and the latest on taxpayer-funded patents, and the latest on the federal innovation ecosphere.
The FedInvent Team
FedInvent tells the stories of inventors, investigators, and innovators. Wayfinder Digital's FedInvent Project follows the federal innovation ecosphere, taxpayer money, and the inventions it pays for.
FedInvent is a work in progress. Please reach out if you have questions or suggestions. You can reach us at info@wayfinder.digital.